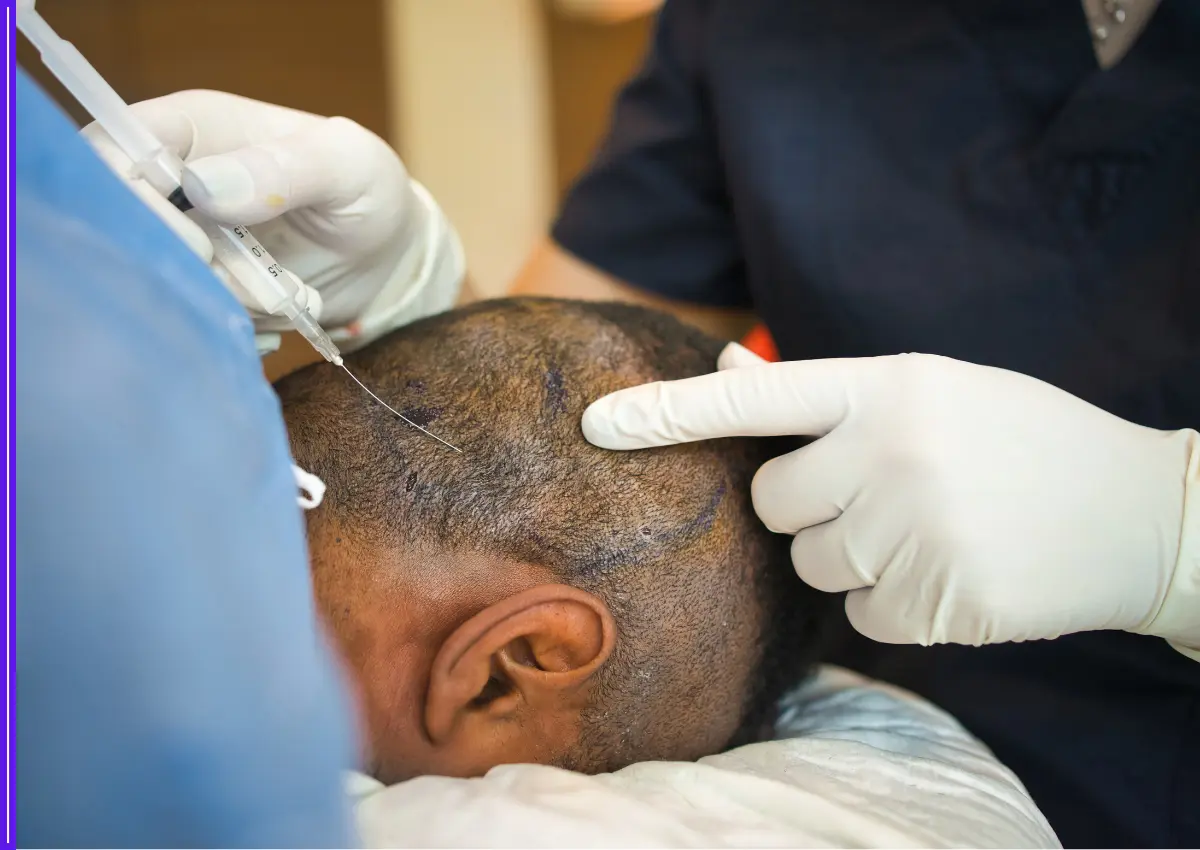
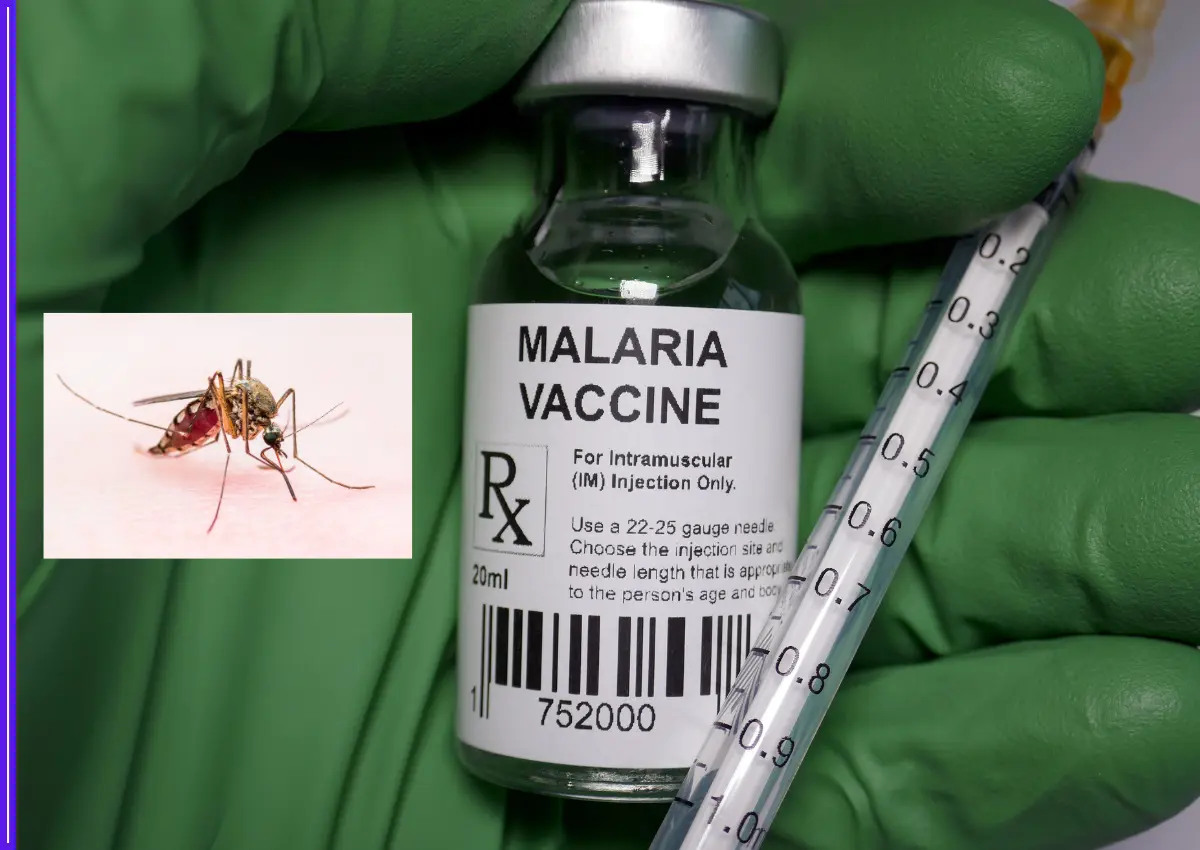
In a significant leap towards indigenous vaccine innovation, India has developed a promising new multi-stage malaria vaccine candidate named AdFalciVax. The vaccine is designed to combat the deadly Plasmodium falciparum parasite by targeting both the infection and transmission stages of the disease.
Developed under the leadership of the Indian Council of Medical Research (ICMR) at its Regional Medical Research Centre in Bhubaneswar, AdFalciVax is being hailed as a potential game-changer in the country’s ongoing battle against malaria. The project is being carried out in collaboration with the ICMR–National Institute of Malaria Research and the Department of Biotechnology’s National Institute of Immunology in New Delhi.
Unlike existing vaccines that typically focus on a single stage of the malaria parasite’s life cycle, AdFalciVax is engineered to act on both the pre-erythrocytic (before the parasite enters the bloodstream) and sexual stages (which allow transmission through mosquitoes). This dual-stage approach is expected to provide better protection against infection and help reduce the spread of malaria within communities.
What makes AdFalciVax particularly significant is its thermal stability. Early trials have shown that the vaccine remains effective even at room temperature for several months—a vital advantage for distribution in India’s rural and tropical regions, where cold-chain logistics remain a challenge.
ICMR has issued an Expression of Interest (EoI), inviting pharmaceutical companies and biotech firms to collaborate on the vaccine’s development and commercialization. Interested organizations will be granted non-exclusive rights to manufacture and market AdFalciVax, with ICMR offering full technical and scientific support throughout the process.
The vaccine is currently undergoing preclinical trials. As per the project timeline, the development and approval process may span up to seven years, with distinct phases covering development, testing, regulatory review, and production readiness. Each phase includes a buffer period to accommodate challenges such as trial delays or regulatory updates.
Officials involved in the project believe AdFalciVax will eventually complement or outperform existing malaria vaccines like RTS,S/AS01 (Mosquirix) and R21/Matrix-M, particularly in terms of broader protection and ease of distribution.
India continues to account for nearly 50% of malaria cases in the WHO South-East Asia region. While national malaria cases have dropped significantly over the past decade, experts caution that innovation in prevention remains crucial to eliminating the disease entirely.
The introduction of an indigenous, affordable, and climate-resilient vaccine like AdFalciVax not only supports India’s public health goals but also aligns with the country’s larger vision of Atmanirbhar Bharat (self-reliant India) in science and healthcare.
Clinical trials for AdFalciVax are expected to begin after successful preclinical validation. If results continue to show promise, India may soon be in a position to export the vaccine to other malaria-endemic countries, especially in Africa and Southeast Asia.